What are the possible side effects of IMCIVREE?
IMCIVREE was well studied and most side effects were generally mild and improved over time
The safety of IMCIVREE has been evaluated in >700 patients over ~10 years of clinical trials
The most common side effects of IMCIVREE include:
- darkening of the skin
- injection site reactions
- nausea
- headache
- diarrhea
- stomach pain
- vomiting
- depression
- erection that happens without any sexual activity in males
These are not all the possible side effects of IMCIVREE. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Most nausea and vomiting events were mild, and none were severe. Nausea and vomiting primarily were reported within the first month of treatment and typically went away after a few days.
Talk to your doctor about how to manage side effects, including nausea and vomiting.
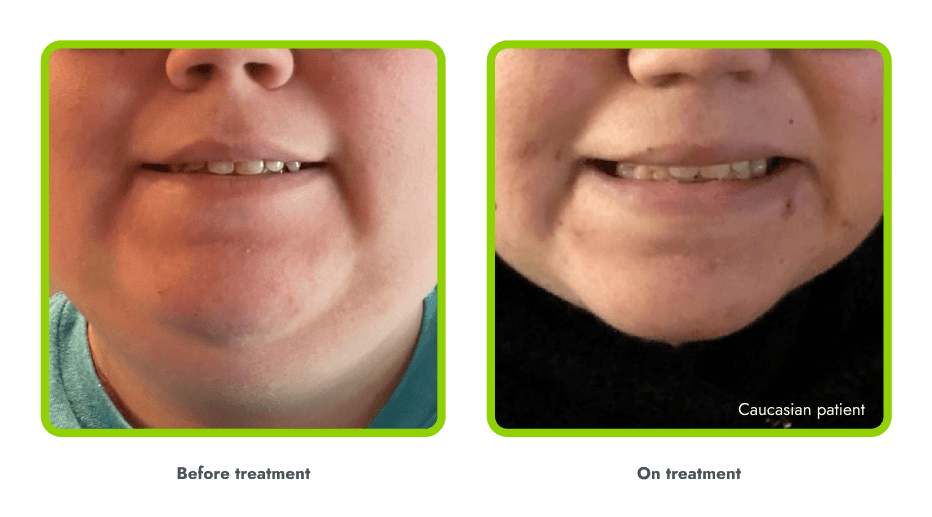
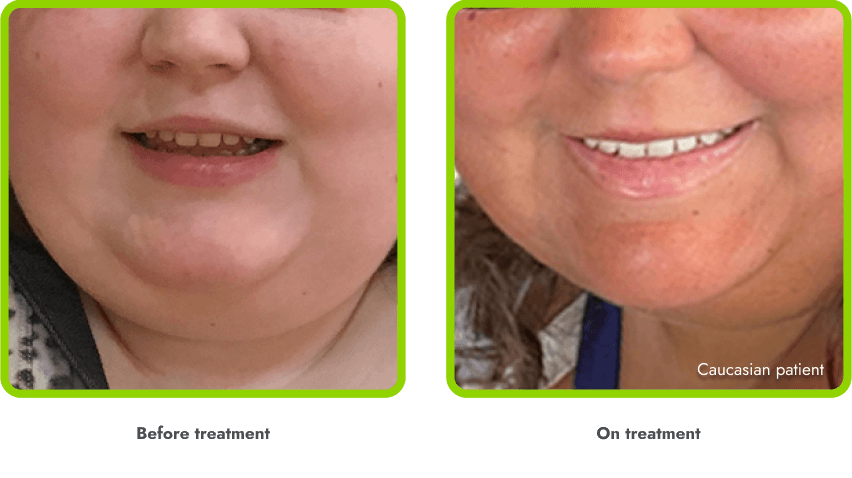
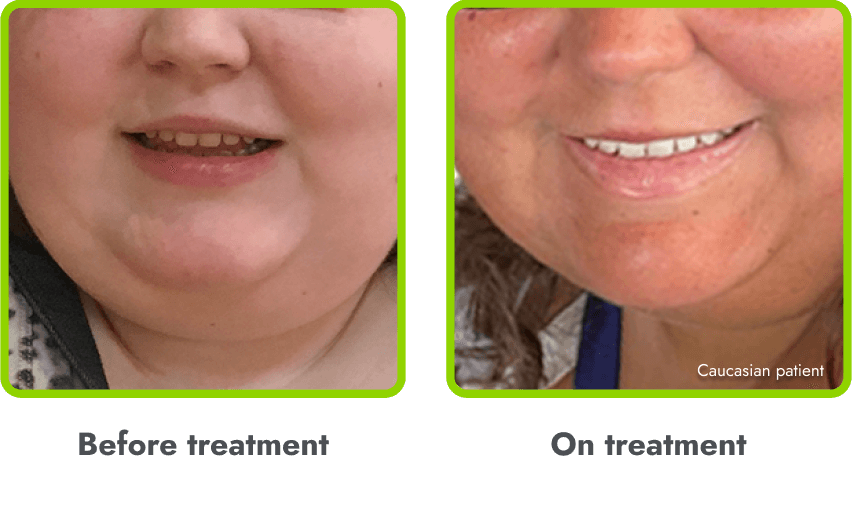
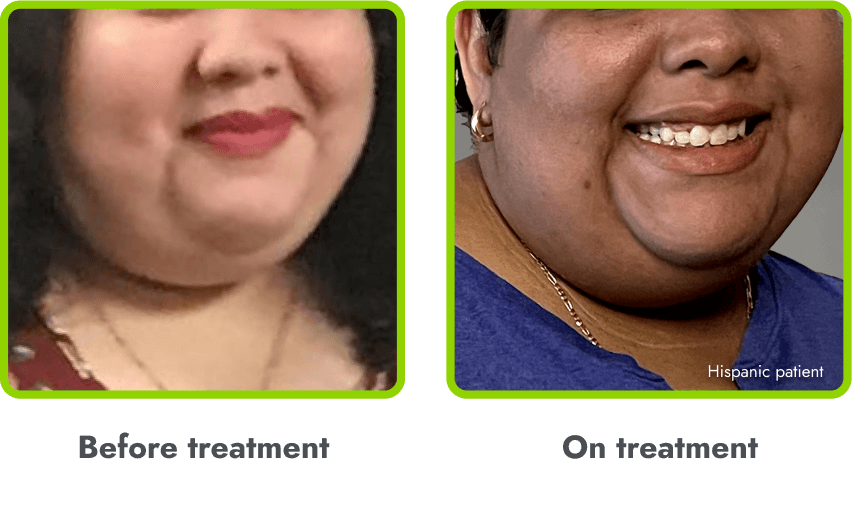
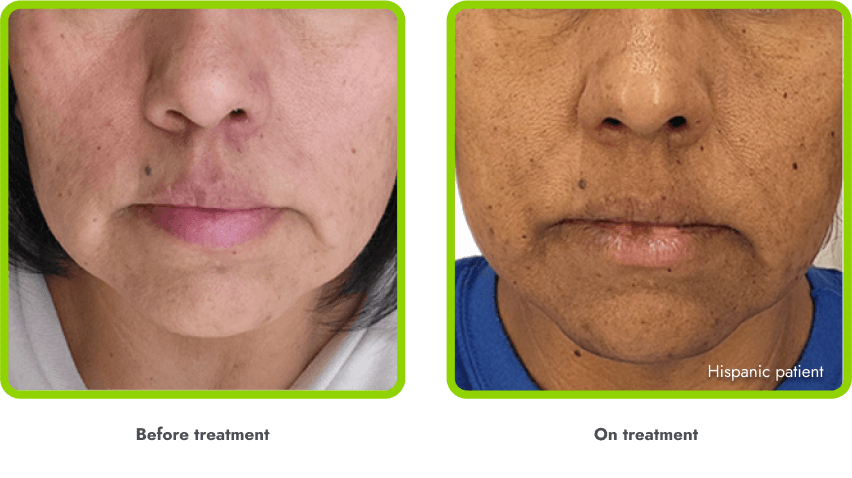
A general darkening of the skin (hyperpigmentation) is common due to the way in which IMCIVREE works
- Darkening of the skin and/or hair is expected, as IMCIVREE may lead to increased production of melanin (which gives color to your skin)
- The degree of skin darkening can vary from person to person, and can sometimes include darkening of existing skin growths (such as moles)
- In the clinical trials, hyperpigmentation increased over the first several weeks of IMCIVREE use and generally leveled off in the initial months of treatment
- Darkening of the skin went away after people stopped using IMCIVREE
If hyperpigmentation is a concern, talk to your doctor, and they will assess your response to treatment and work with you to create a plan to check for skin changes.
You should have a full-body skin exam before starting and during treatment with IMCIVREE to check for skin changes.
These are not all the possible side effects of IMCIVREE. Please review all the possible side effects of IMCIVREE included in the Patient Information and talk to your doctor about any questions you may have.
Managing possible side effects with IMCIVREE
Hear from healthcare providers who have experience managing their patients' side effects
*Patient Education Managers are employees of Rhythm Pharmaceuticals and do not provide medical care or advice.
We encourage you to always speak to your healthcare providers regarding your medical care.