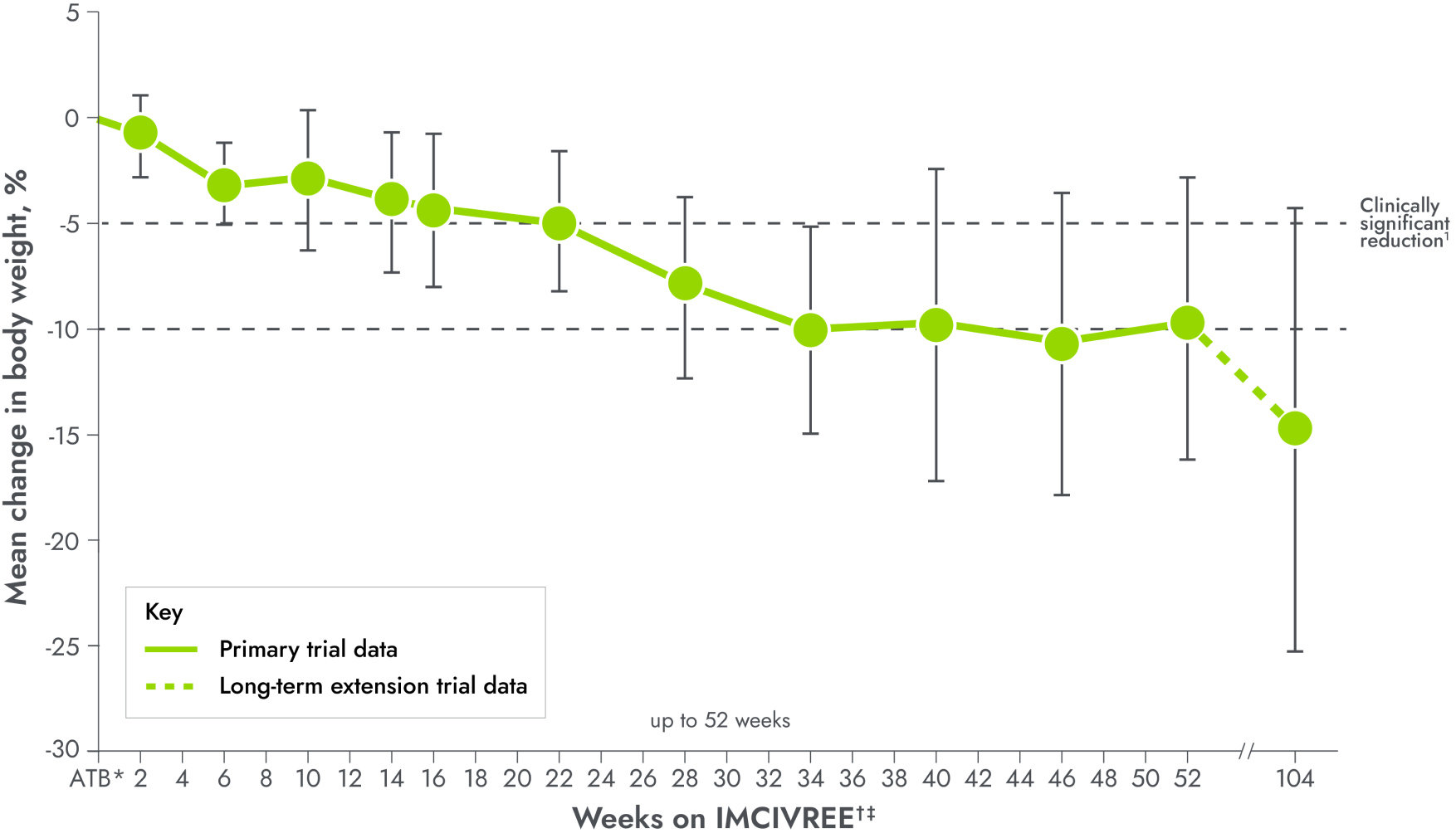
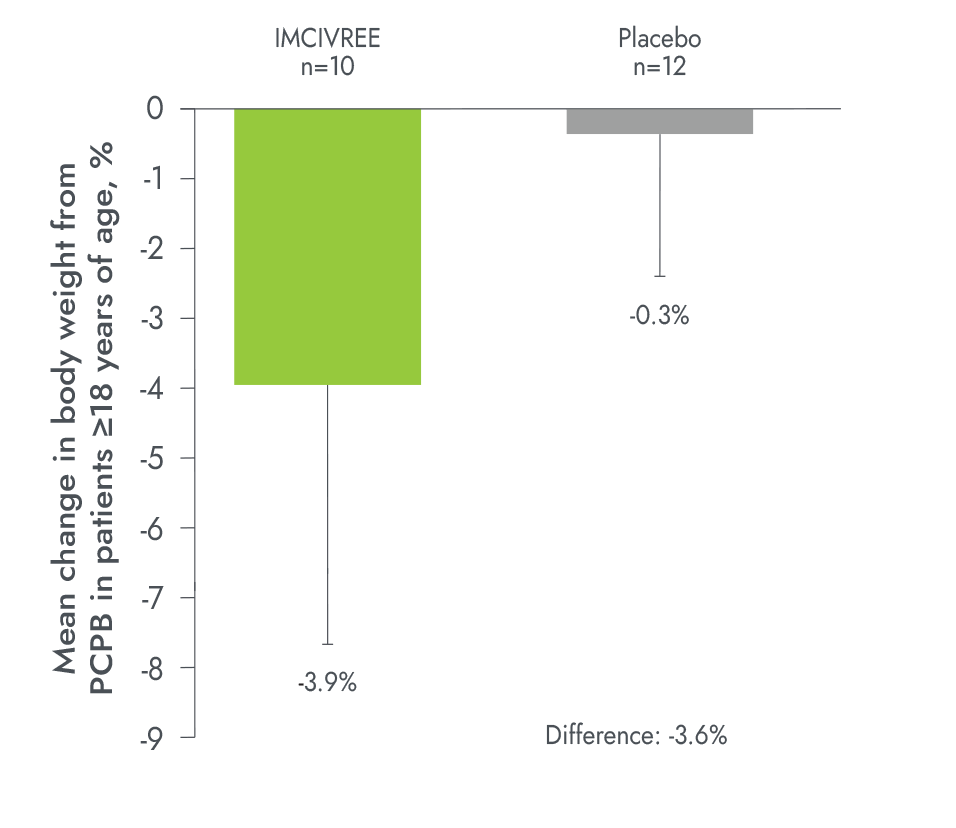
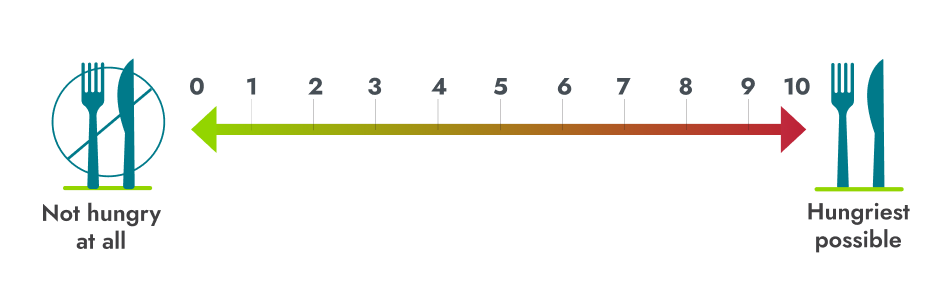IMCIVREE delivered early, significant, and sustained weight reduction1,2
Percentage change in weight in patients ≥18 years of age after 52 weeks (n=12)4
Patients were not required to change their diet or exercise routine2
*Patients with data after 52 weeks of treatment.4
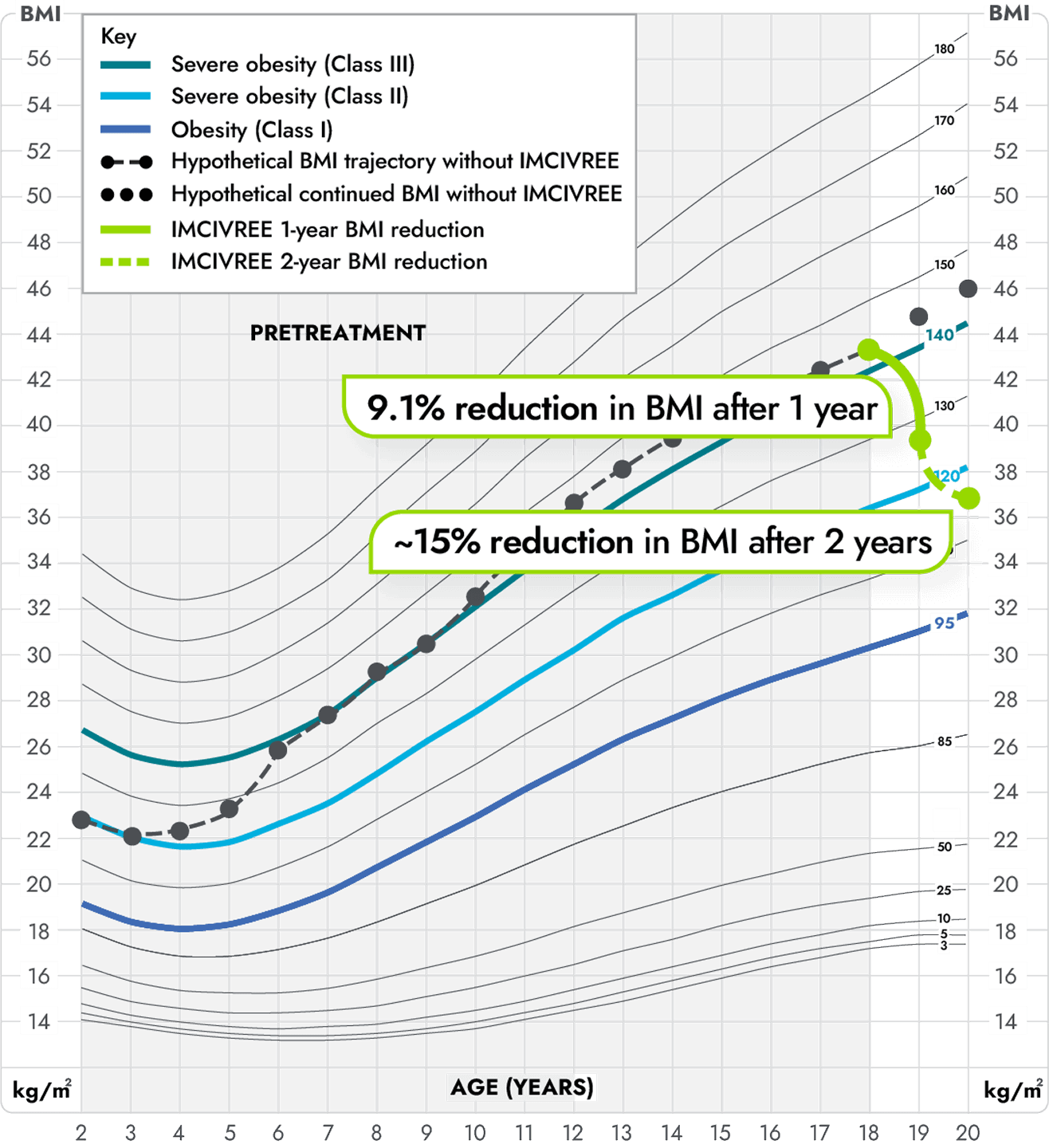
Figure modeled after Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130(6):1136-1140.5
*Not an actual patient.
†Patients with data after 1 year of treatment.3
‡Growth chart is based on females 2 to 20 years of age and is for illustrative purposes only.
In patients ≥12 years of age with BBS
Hunger scores in the 14-week placebo-controlled and 52-week open-label periods6,7*
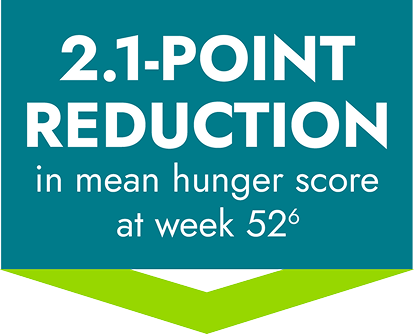
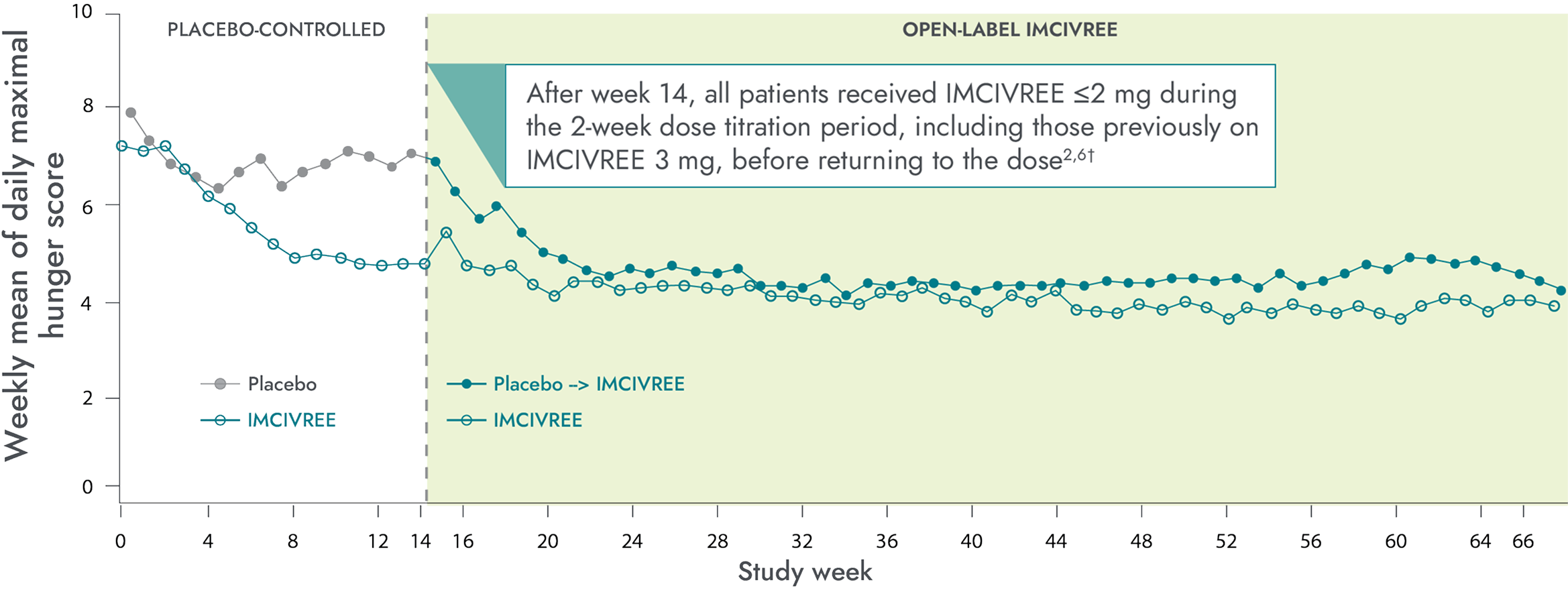
Patients who switched from placebo to IMCIVREE experienced a rapid reduction in hunger, matching that of patients initially assigned to IMCIVREE4
Caregiver assessments were also collected for children <12 with obesity due to BBS‡
*Patients ≥12 years of age who were able to self-report their hunger (n=14) recorded their daily maximal hunger in a diary, which was then assessed by the Daily Hunger Questionnaire Item 2. Hunger was scored on an 11-point scale from 0 (“not hungry at all”) to 10 (“hungriest possible”).6
†During the placebo-controlled period, dose titration to a fixed dose of IMCIVREE 3 mg given subcutaneously once daily was performed during the first 2 weeks of both the placebo-controlled and open-label periods to maintain blinding.6
‡Data not shown.

Before IMCIVREE, I didn’t realize how much time I spent focusing on food, and how much that was affecting my day-to-day and the other things I could be accomplishing.
— Kathryn, who is living with BBS

Patients and families living with BBS often express such gratitude and relief when started on IMCIVREE and see the weight trajectories and hyperphagia start to improve. To have a medication that can support correcting the biology that is dysregulated and to see positive outcomes provides hope.
— Alaina Vidmar, Pediatric Endocrinologist

These are patients that nothing else has ever worked. So to see them so happy about having something that's finally helping is amazing. Now all they talk about is, ’I'm not hungry all the time. I'm not seeking out food all the time. I'm able to do other things.‘ That shift from the number on the scale to the quality of life, I really love that.
— Christy Davis, Obesity and Weight Management Specialist
Individual results may vary.
Patient-Reported Health-Related Quality of Life
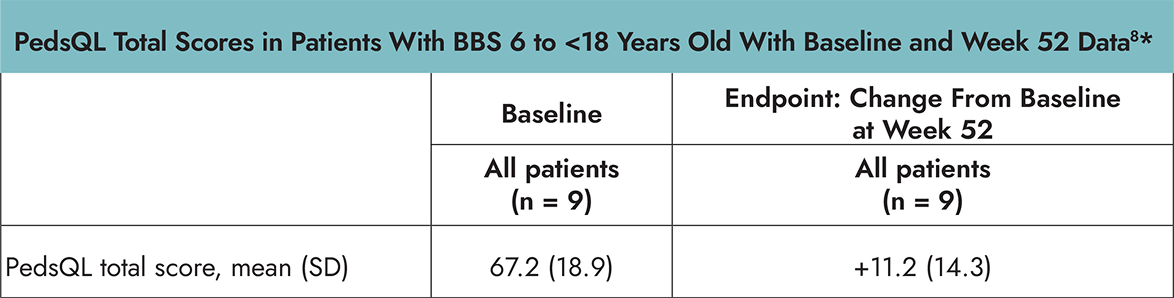
In the Phase 3 trial in patients 6 years and older with BBS, PedsQL and IWQOL-Lite were assessed as exploratory endpoints and were not powered for formal statistical testing or significance. Change from baseline after approximately 52 weeks of treatment was measured by the age-specific PedsQL or IWQOL-Lite assessments.8
- The PedsQL is a 23-item, self-reported, age-dependent assessment of health-related quality of life (HRQOL) in children and adolescents with or without acute or chronic health conditions that encompasses 4 domain scores: physical, emotional, social, and school functioning. The total score is the mean score of the transformed items across the 4 domains. The PedsQL was administered to children 6 to <18 years of age.8,9
- The Impact of Weight on Quality of Life (IWQOL)-Lite is a validated 31-item, self-reported, obesity specific, quality of life questionnaire that provides a total score inclusive of 5 domains: physical function, self-esteem, sexual life, public distress, and work. The IWQOL-Lite was administered to patients ≥18 years of age.8
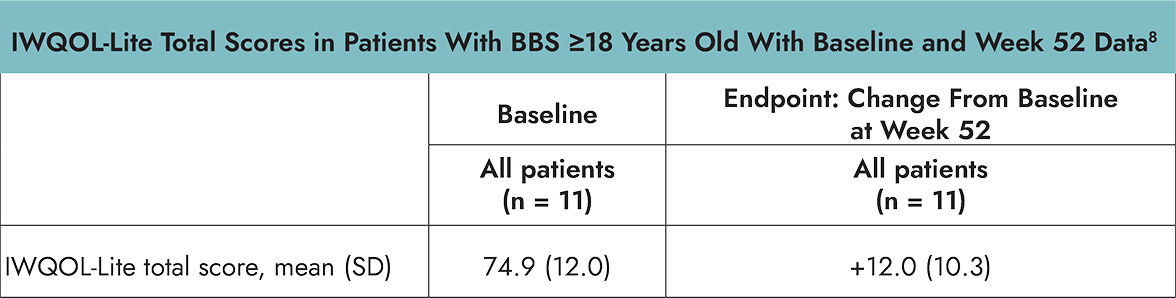
- Raw scores for both PedsQL and IWQOL-Lite were transformed on a scale of 0–100, with 0 representing the worst possible and 100 the best possible HRQOL.8
- Limitations of these results include small sample sizes across assessments, which may be in part due to the rarity of the disease.8
These insights highlight the need to address hyperphagia and subsequent impaired quality of life for people with BBS and their caregivers10
*Using age-specific PedsQL or IWQOL-Lite assessments.8
HRQoL=health-related quality of life; IWQOL-Lite=Impact of Weight on Quality of Life-Lite; PedsQL=Pediatric Quality of Life Inventory.
The impact of IMCIVREE
Hear from families and clinicians about how IMCIVREE is bringing hope for people living with BBS

References: 1. US Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(23):2417-2426. doi:10.1001/jama.2017.6803 2. Haqq AM, Chung WK, Dollfus H, et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022;10(12):859-868. doi:10.1016/S2213-8587(22)00277-7. 3. Argente J et al. Endocrine Society Annual Meeting. Poster ODP606. June 11-14, 2022. 4. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA 5. Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics. 2012;130(6):1136-1140. doi:10.1542/peds.2012-0596. 6. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc. 7. Haqq AM et al. Lancet Diabetes Endocrinol. 2022;10(12):859-868. doi:10.1016/S2213-8587(22)00277-7. Supplementary appendix. 8. Forsythe E, Haws RM, Argente J, et al. Quality of life improvements following one year of setmelanotide in children and adult patients with Bardet-Biedl syndrome: phase 3 trial results. Orphanet J Rare Dis. 2023;18(1):12. Published 2023 Jan 16. doi:10.1186/s13023-022-02602-4. 9. Varni JW. Scaling and scoring for the acute and standard versions of the Pediatric Quality of Life Inventory™ PedsQL™. Version 21.3. March 2023. Accessed December 15, 2024. https://www.pedsql.org/PedsQL-Scoring.pdf. 10. Eneli I, Xu J, Webster M, et al. Tracing the effect of the melanocortin-4 receptor pathway in obesity: study design and methodology of the TEMPO registry. Appl Clin Genet. 2019;12:87-93. doi:10.2147/TACG.S199092.