Only IMCIVREE was studied across two, first-ever Phase 3 clinical trials dedicated to obesity and hunger reduction in people with BBS1-4
The efficacy and safety of IMCIVREE for the reduction of weight and hunger in patients with BBS were studied in a Phase 3 trial with a randomized, double-blind, placebo-controlled period1,4
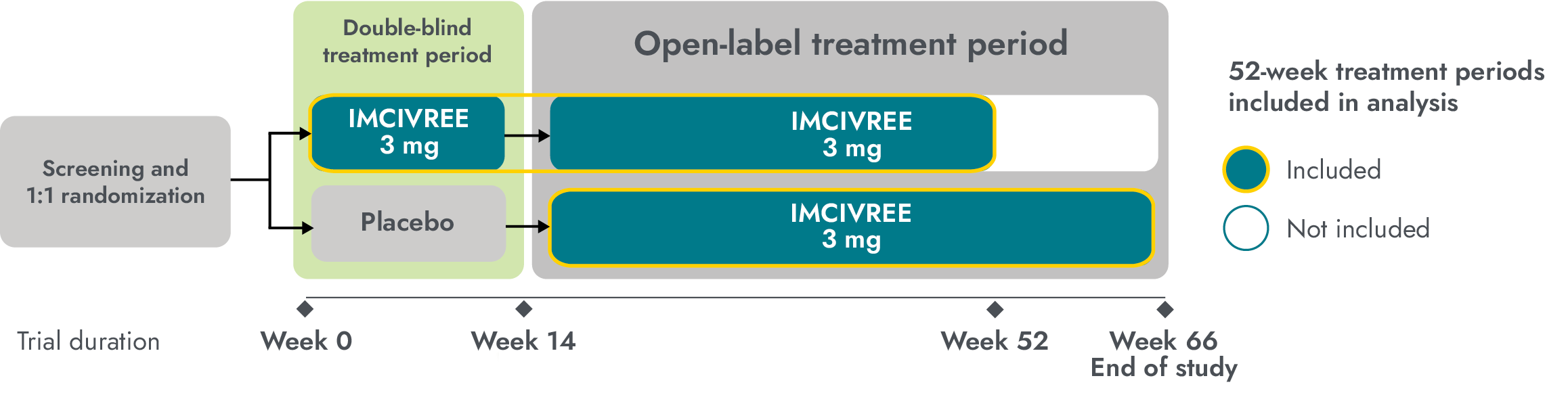
Study design: The study enrolled patients ≥6 years of age with obesity and a clinical diagnosis of BBS. Adult patients had a BMI of ≥30 kg/m2 and pediatric patients had weight in the ≥97th percentile using growth chart assessments. To maintain the blind during period 1 (14-week placebo-controlled period), dose titration to a fixed dose of 3 mg given subcutaneously once daily was performed during the first 2 weeks of both period 1 and period 2 (52-week open-label period). Efficacy analyses were conducted in 44 patients at the end of period 1 (week 14, placebo-controlled data) and in 31 patients during the active-treatment period, defined as the period from randomization to week 52 in patients initially randomized to IMCIVREE, and from week 14 to week 66 in patients initially randomized to placebo. Analyses of the active-treatment period include patients who had either completed 52 weeks from the start of IMCIVREE treatment or discontinued the study early at the time of the prespecified data cutoff.1
IMCIVREE delivered BMI reductions3
~10%
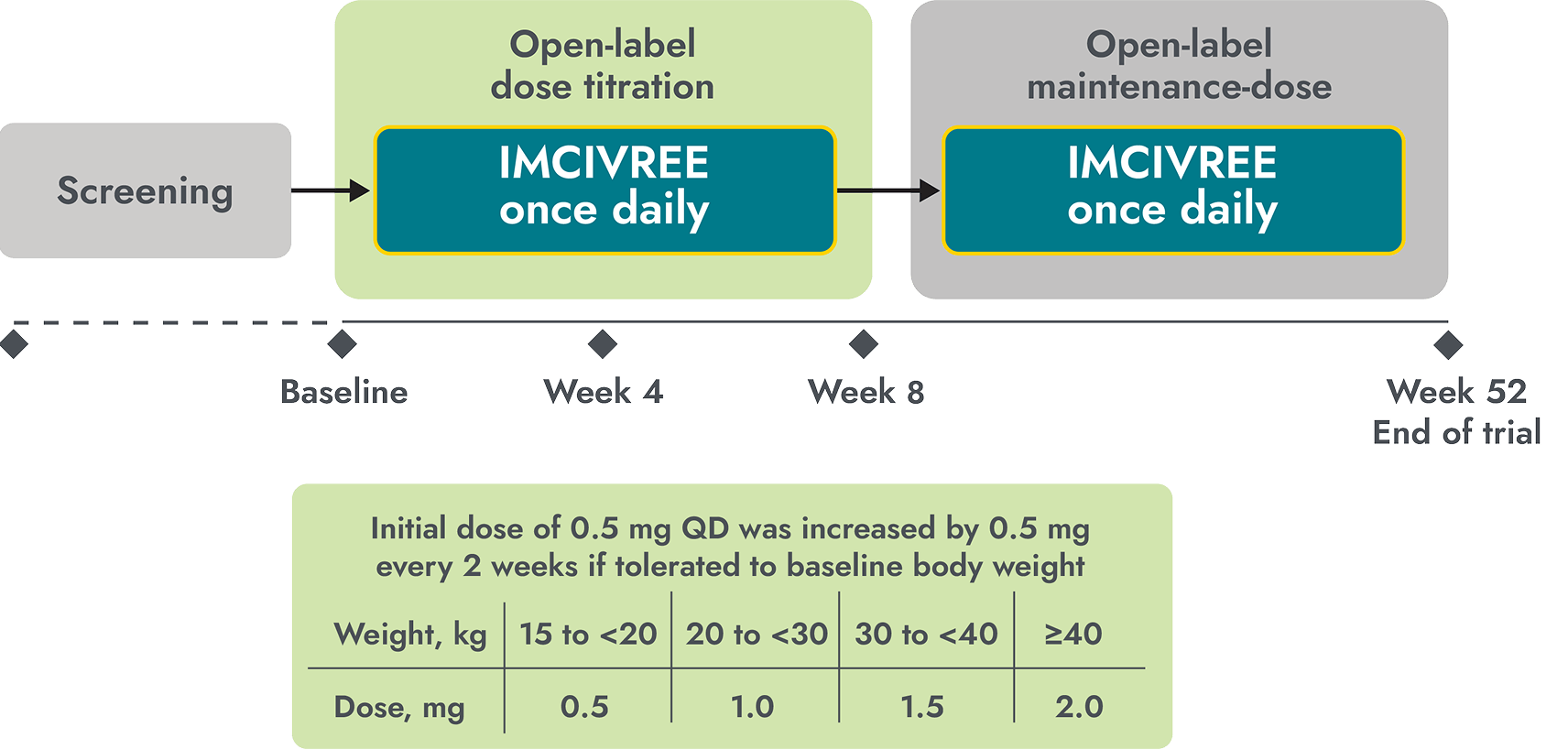
mean BMI reduction after 1 year in young children aged 2 to <6 years2
~8%
mean BMI reduction after 1 year in children and adults aged ≥6 years1
Patients were not required to change their diet or exercise routine3

Supportive of the effect of IMCIVREE on weight loss, there were general numeric improvements in blood pressure, lipids, and waist circumference. However, because of the limited number of patients studied and the lack of a control group the treatment effects on these parameters could not be accurately quantified.1,2

IMCIVREE is
foundational to the
long-term treatment of
obesity due to BBS as the
only precision medicine
designed to re-establish MC4R
pathway activity. This pathway
activity is critical to maintain
regulated satiety signaling,
energy expenditure,
and weight1