Once-daily, subcutaneous injection that can be administered at home1
Titrate patients to the maintenance dose to optimize efficacy and tolerability


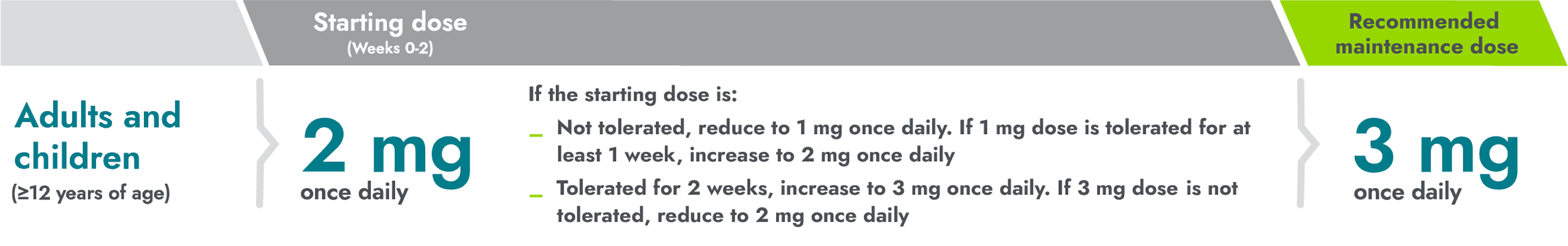
- IMCIVREE should be administered once daily, at the beginning of the day, without regard to meals1
- There is no food requirement for administration1
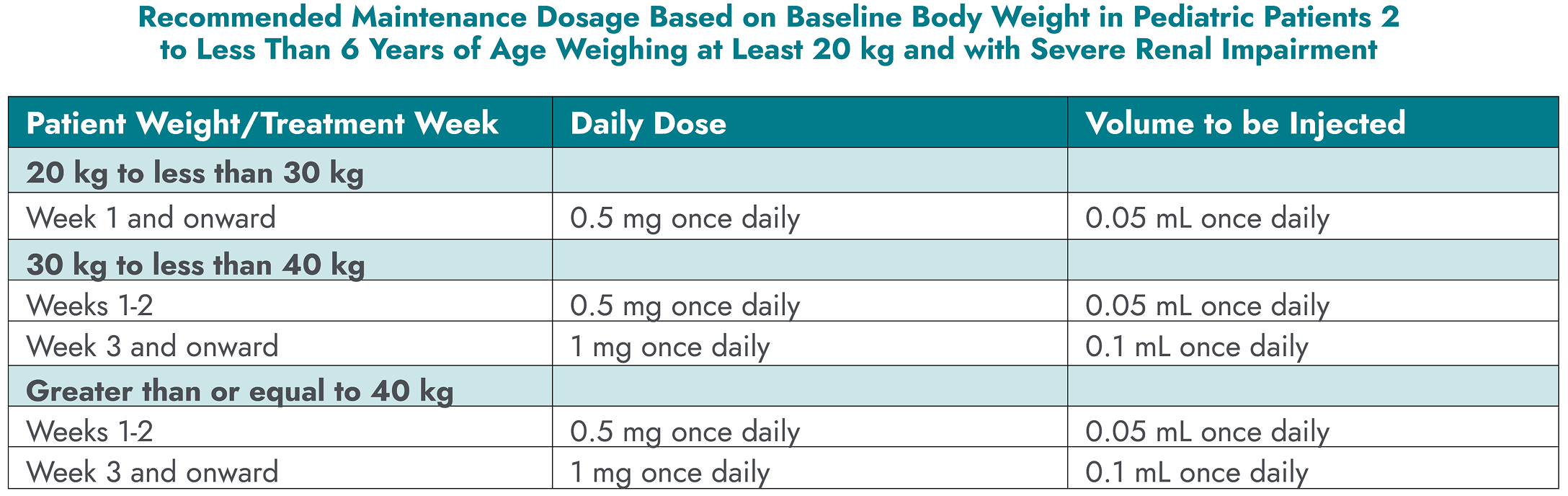
- No dose adjustments are needed for patients with mild to moderate renal impairment1

10-mg/1-mL
multiple-dose vial
Effective, long-term weight management starts with setting patient expectations
Achieving the benefits of treatment with IMCIVREE may take time
In clinical trials, it took some time for hunger and weight reductions to occur, while patients experienced certain AEs soon after starting treatment. This chart highlights some of the most common AEs, but it does not include all reported AEs.
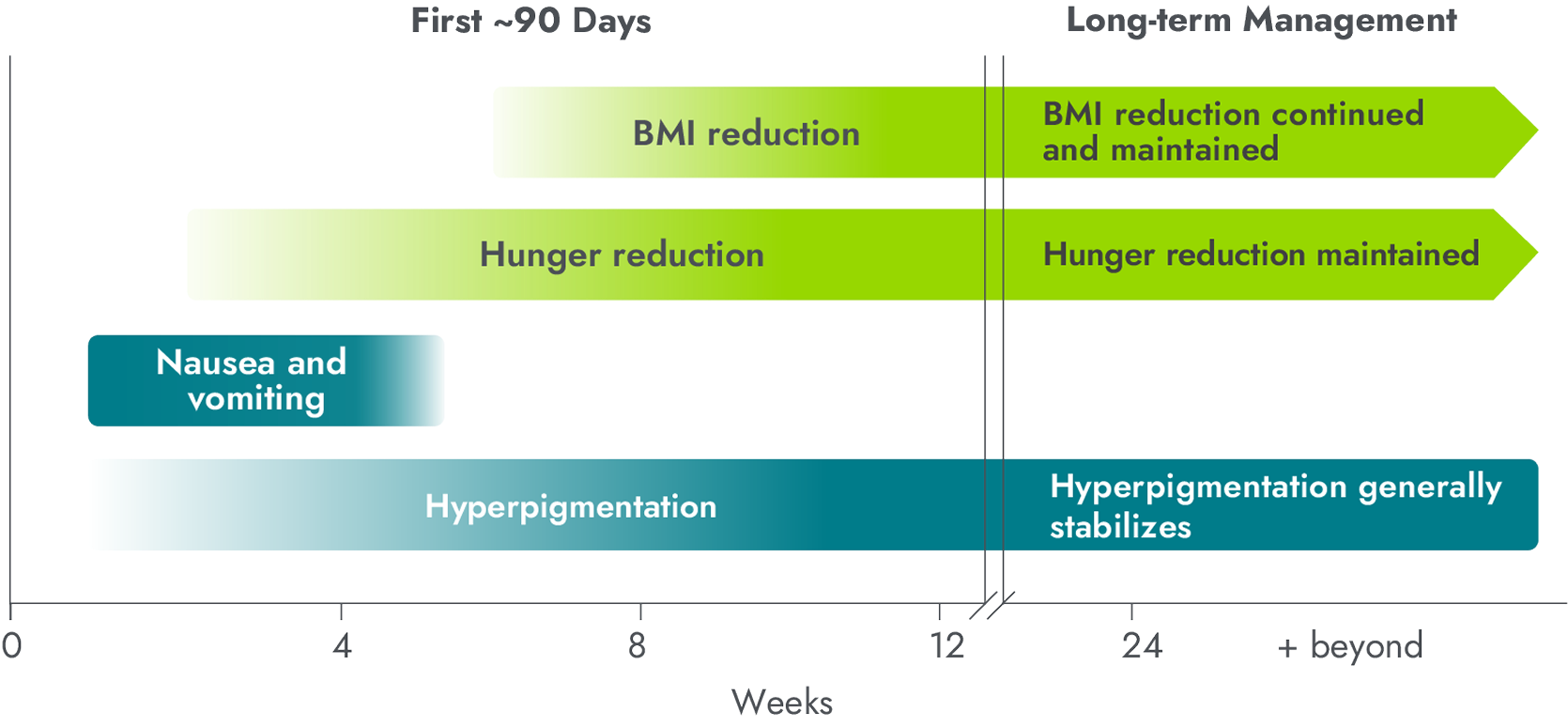
- Meaningful weight reduction began within 6-8 weeks and continued over time 1-5
- Hunger reduction began within weeks. Measures of hunger increased quickly upon dose reduction or discontinuation of IMCIVREE1-3,6
- Nausea and vomiting were primarily reported within the first 4 weeks of treatment and typically resolved within a few days; nearly all events were mild and none were serious2,3,5,6
- Hyperpigmentation typically occurred within 2-3 weeks and increased throughout the dose titration period; levels generally plateaued in the initial months of treatment and were sustained over long-term treatment with IMCIVREE6
Managing patient expectations
In clinical trials:
Talking to patients about when they can expect weight and hunger benefits and common adverse reactions may help them manage through the short-term treatment initiation to achieve their longer-term goals
AE=adverse event.