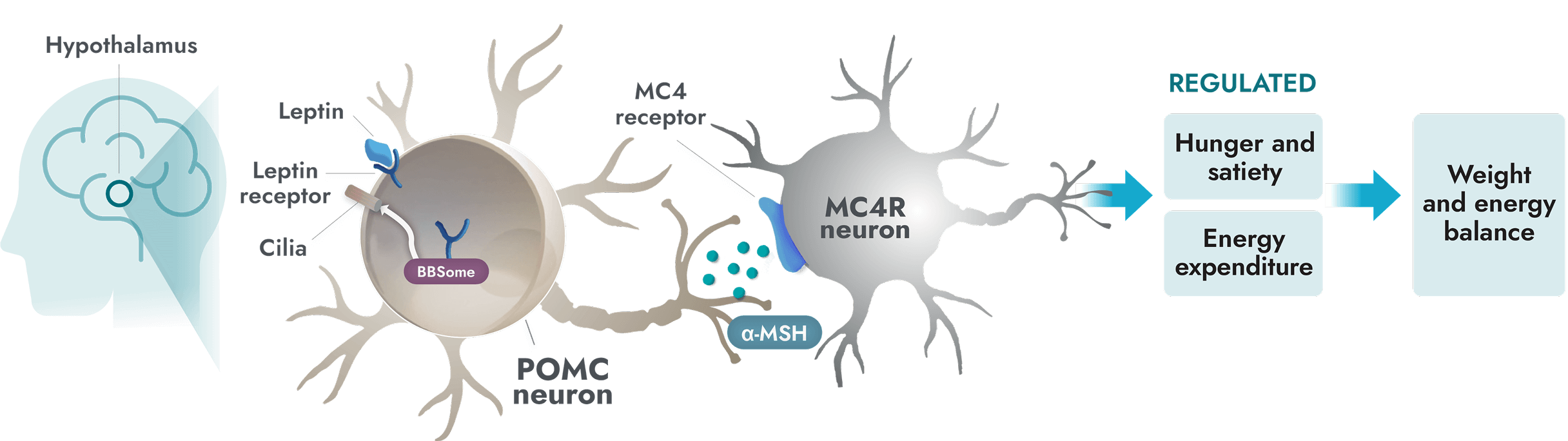
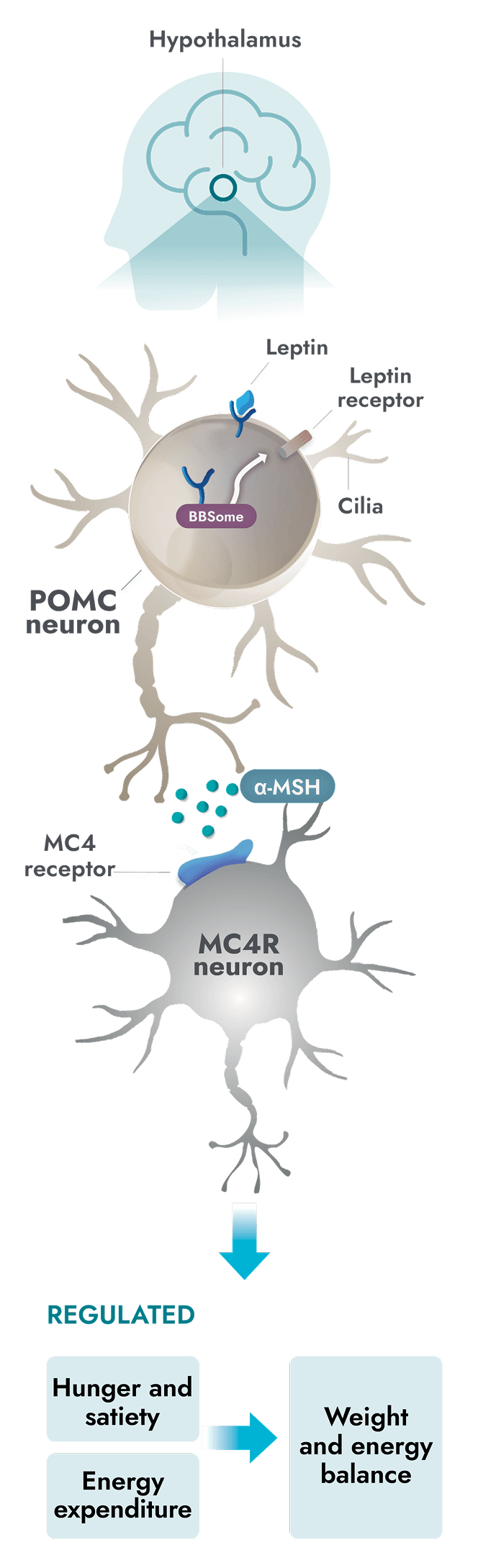
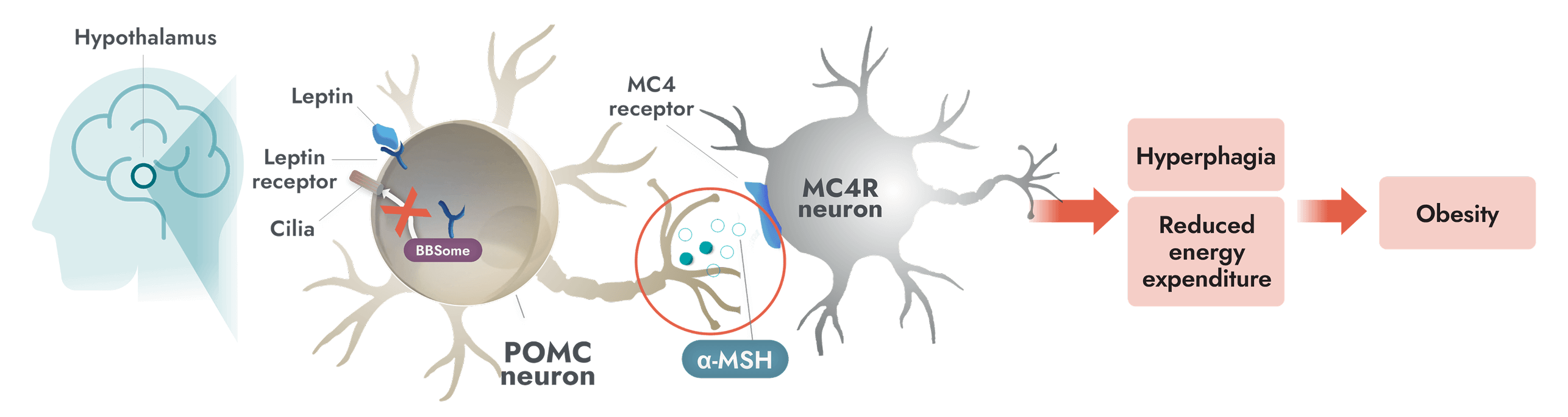
The MC4R pathway is a key signaling pathway in the hypothalamus that regulates hunger and energy expenditure1
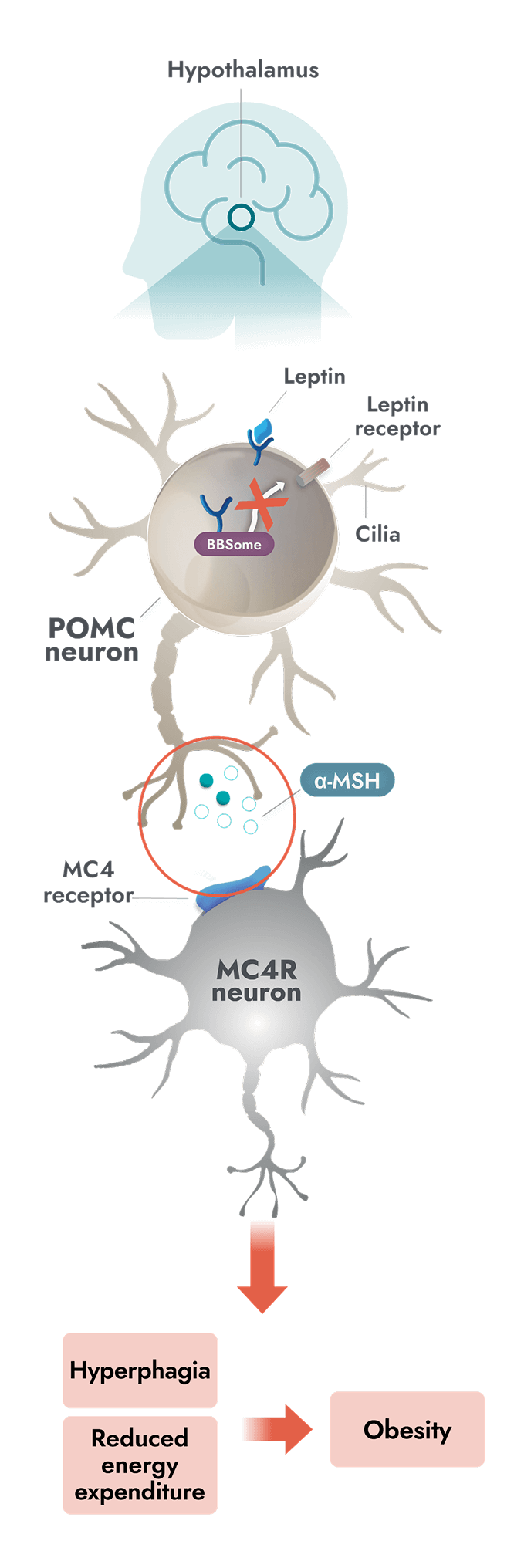
Unlike general obesity, a root cause of obesity due to BBS is impairment of the MC4R pathway which can occur due to ciliary dysfunction1
- In people with BBS, a variant in one or more BBS genes can disrupt the BBSome. This causes ciliary dysfunction and disruption of LEPR signaling2,7
- Alpha-melanocyte-stimulating hormone (α-MSH) production is impaired or deficient, preventing activation of the MC4 receptor1
- Impairment of the MC4R pathway leads to decreased satiety signaling, hyperphagia, and reduced energy expenditure. This often leads to early-onset obesity1
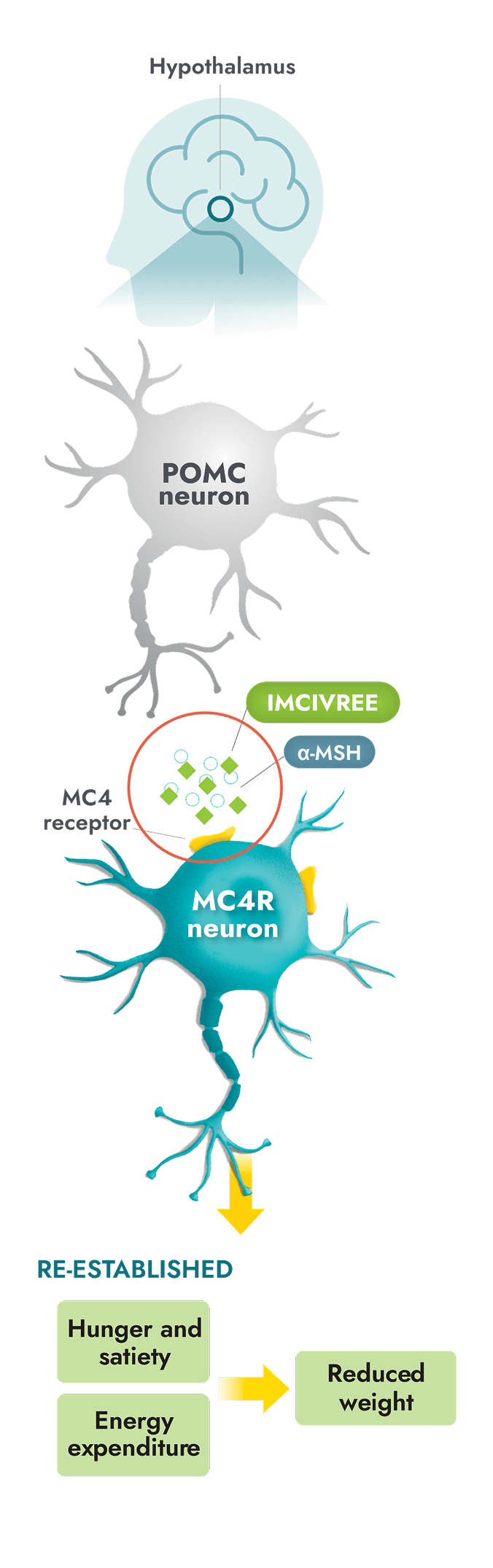
IMCIVREE is the first and only precision medicine to target impairment of the hypothalamic MC4R pathway, a root cause of hyperphagia and obesity due to BBS1,8
- IMCIVREE, an MC4R agonist, targets a root cause of obesity due to BBS8-10
- IMCIVREE acts in place of alpha-melanocyte-stimulating hormone (α-MSH) to activate the MC4 receptor and help re-establish MC4R pathway activity8-10
- Re-established pathway activity helps to bring hunger and satiety signals, and energy expenditure, into balance. This can lead to reduced weight8
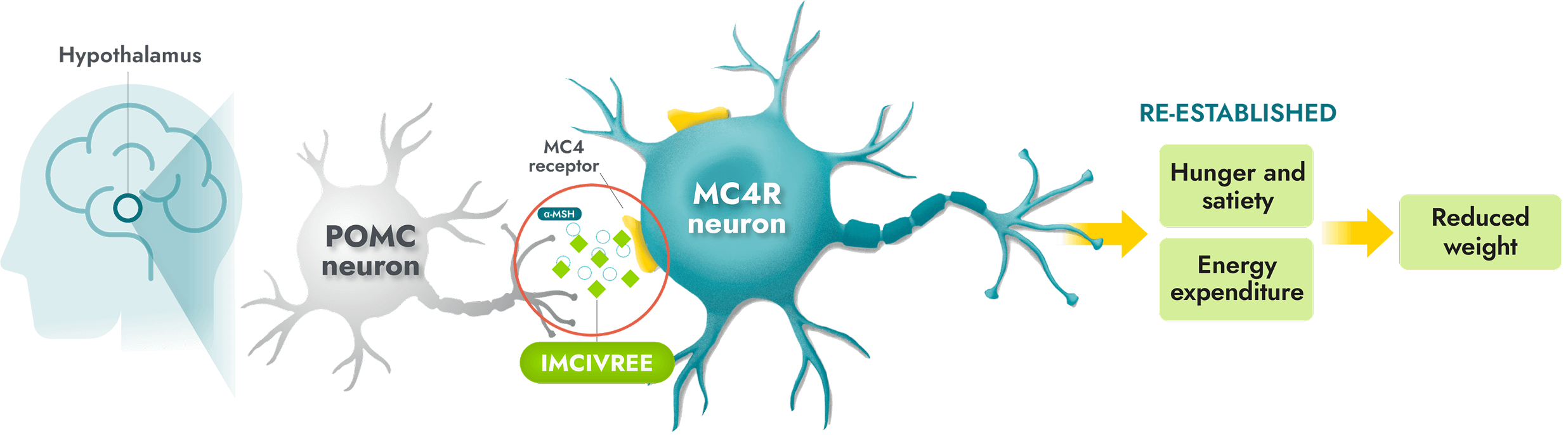
IMCIVREE is foundational to the long-term treatment of obesity due to BBS. Continuous treatment with IMCIVREE helps re-establish and maintain MC4R pathway activity8,11
MC4R=melanocortin-4 receptor; POMC=proopiomelanocortin.
References: 1. Eneli I, Xu J, Webster M, et al. Tracing the effect of the melanocortin-4 receptor pathway in obesity: study design and methodology of the TEMPO registry. Appl Clin Genet. 2019;12:87-93. doi:10.2147/TACG.S199092. 2. Blaess S, Wachten D. The BBSome: a nexus controlling energy metabolism in the brain. J Clin Invest. 2021;131(8):e148903. doi:10.1172/JCI148903. 3. Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323-1331. doi:10.1093/hmg/ddp031. 4. Ciliopathy Alliance. Cilia. 2024. Accessed December 11, 2024. https://ciliopathyalliance.org/cilia. 5. Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia. 2012;1(1):4. doi:10.1186/2046-2530-1-4. 6. National Cancer Institute. NCI Dictionary of Cancer Terms. Accessed December 11, 2024. https://www.cancer.gov/publications/dictionaries/cancer-terms/. 7. Huvenne H, Dubern B, Clément K, Poitou C. Rare Genetic Forms of Obesity: Clinical Approach and Current Treatments in 2016. Obes Facts. 2016;9(3):158-173. doi:10.1159/000445061. 8. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc. 9. Trapp CM, Censani M. Setmelanotide: a promising advancement for pediatric patients with rare forms of genetic obesity. Curr Opin Endocrinol Diabetes Obes. 2023;30(2):136-140. doi:10.1097/MED.0000000000000798. 10. Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020;22(11):2133-2140. doi:10.1111/dom.14133. 11. Haqq AM et al. Lancet Diabetes Endocrinol. 2022;10(12):859-868. doi:10.1016/S2213-8587(22)00277-7.