Patient-Reported Health-Related Quality of Life
In the Phase 3 trial in patients with BBS, PedsQL and IWQOL-Lite were assessed as exploratory endpoints and were not powered for formal statistical testing or significance. Change from baseline after approximately 52 weeks of treatment was measured by the age-specific PedsQL or IWQOL-Lite assessments.1
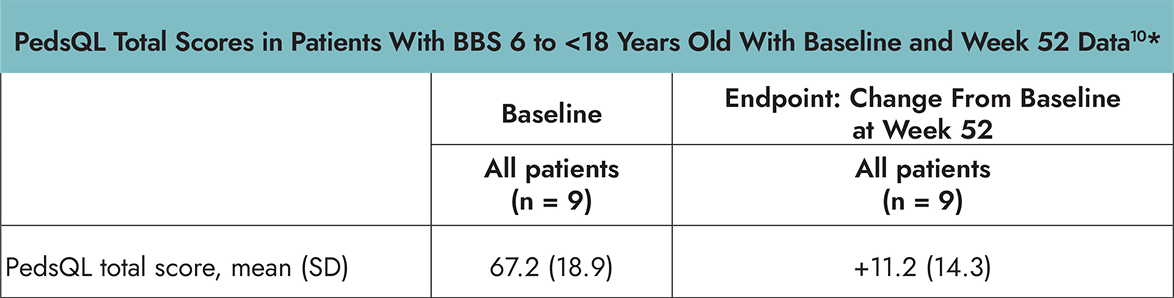
- The PedsQL is a 23-item, self-reported, age-dependent assessment of health-related quality of life (HRQOL) in children and adolescents with or without acute or chronic health conditions that encompasses 4 domain scores: physical, emotional, social, and school functioning. The total score is the mean score of the transformed items across the 4 domains. The PedsQL was administered to children <18 years of age.1,2
- The Impact of Weight on Quality of Life (IWQOL)-Lite is a validated 31-item, self-reported, obesity specific, quality of life questionnaire that provides a total score inclusive of 5 domains: physical function, self-esteem, sexual life, public distress, and work. The IWQOL-Lite was administered to patients ≥18 years of age.1,3
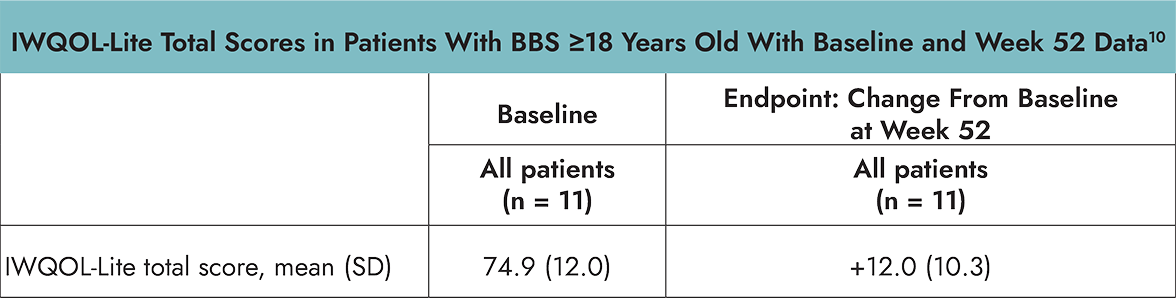
- Raw scores for both PedsQL and IWQOL-Lite were transformed on a scale of 0–100, with 0 representing the worst possible and 100 the best possible HRQOL.3,4
- Limitations of these results include small sample sizes across assessments, which may be in part due to the rarity of the disease.1
These insights highlight the need to address hyperphagia and subsequent impaired quality of life for people with BBS and their caregivers5
*Using age-specific PedsQL or IWQOL-Lite assessments.1
HRQoL=health-related quality of life; IWQOL-Lite=Impact of Weight on Quality of Life-Lite; PedsQL=Pediatric Quality of Life Inventory.
The impact of IMCIVREE
Hear from families and clinicians about how IMCIVREE is bringing hope for people living with BBS

HRQoL=health-related quality of life; IWQOL-Lite=Impact of Weight on Quality of Life-Lite; PedsQL=Pediatric Quality of Life Inventory.