IMCIVREE was studied across three open-label, Phase 3 clinical trials in patients with POMC, PCSK1, or LEPR deficiency1-3
IMCIVREE was studied in the first-ever Phase 3 clinical trial for children 2 to <6 years of age with obesity due to POMC, PCSK1, or LEPR deficiency1,2,4*

Endpoints:
- Mean percent change in BMI from baseline to Week 52 (primary endpoint)
- Proportion of participants with a decrease in BMI Z-score of ≥0.2 at Week 52 (POMC, N=3; LEPR, N=4)
*Efficacy and safety analyses were conducted at the end of treatment in 12 patients (7 with POMC or LEPR deficiency and 5 with BBS). Patients with PCSK1 deficiency were eligible but none enrolled in the trial.
IMCIVREE was studied in 2 identically designed, 1-year, open-label studies, each with an 8-week, double-blind withdrawal period in patients 6 years and older with obesity due to POMC, PCSK1, and LEPR deficiency1,3
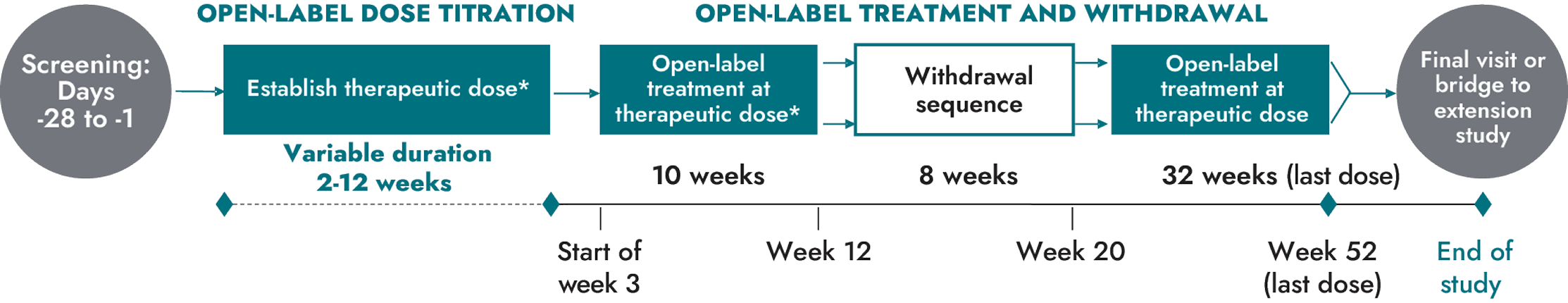
Primary endpoint:
- Proportion of participants who achieve ≥10% weight loss at 1 year of treatment (POMC or PCSK1, N=10; LEPR, N=11)
*Only participants who lost ≥5 kg weight (or ≥5% of body weight if baseline weight was <100 kg) in the first open-label active treatment phase entered an 8-week, double-blind withdrawal period.
Baseline characteristics in these IMCIVREE trials1,3,4
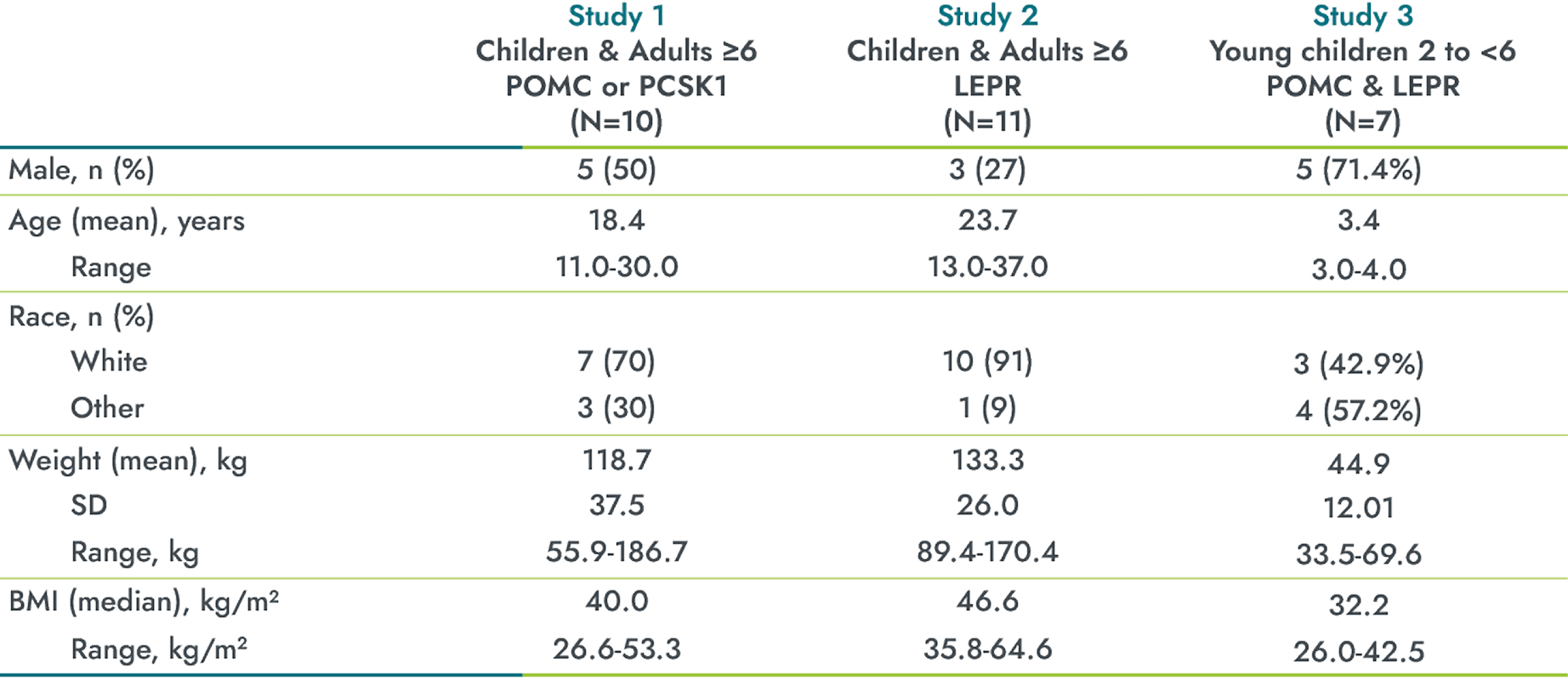
*LEPR=leptin receptor; PCSK1=proprotein convertase subtilisin/kexin type 1;
POMC=proopiomelanocortin; SD=standard deviation.
References: 1. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc. 2. Argente J, Verge CF, Okorie U, et al. Setmelanotide in patients aged 2–5 years with rare MC4R pathway-associated obesity (VENTURE): a 1 year, open-label, multicenter, phase 3 trial. Lancet Diabetes Endocrinol. 2024;24:1-18. doi: 10.1016/s2213-8587(24)00273-0. 3. Clément K, van den Akker E, Argente J, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960-970. doi:10.1016/S2213-8587(20)30364-8. 4. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA.