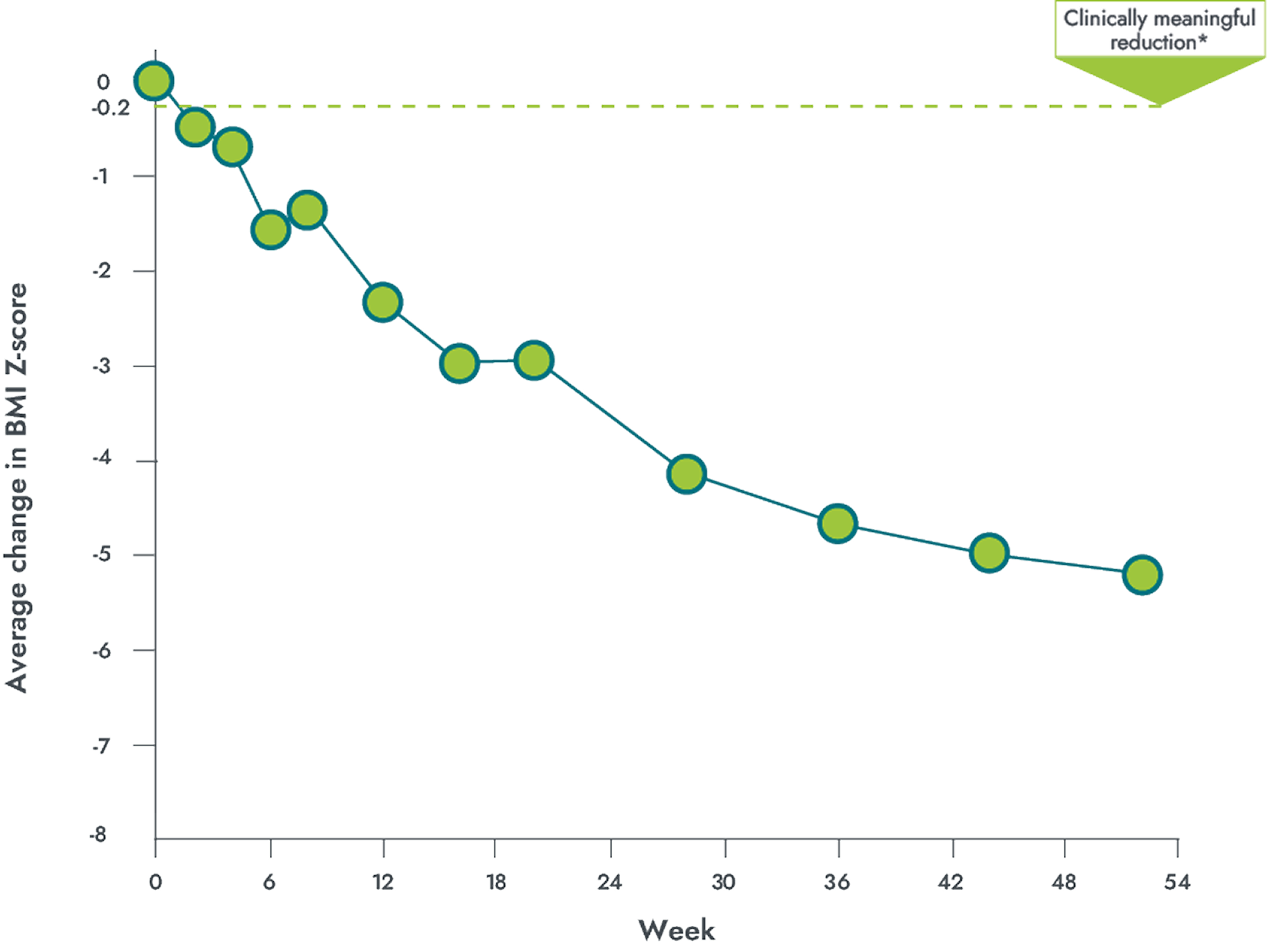
In patients with obesity due to POMC, PCSK1, or LEPR deficiency
IMCIVREE delivered clinically meaningful weight loss over 1 year1,2
IMCIVREE delivered significant, clinically meaningful weight loss over 1 year in patients 6 years and older with POMC or PCSK1 deficiency
Mean % change in body weight over 1 year
POMC/PCSK1 (n=9)*
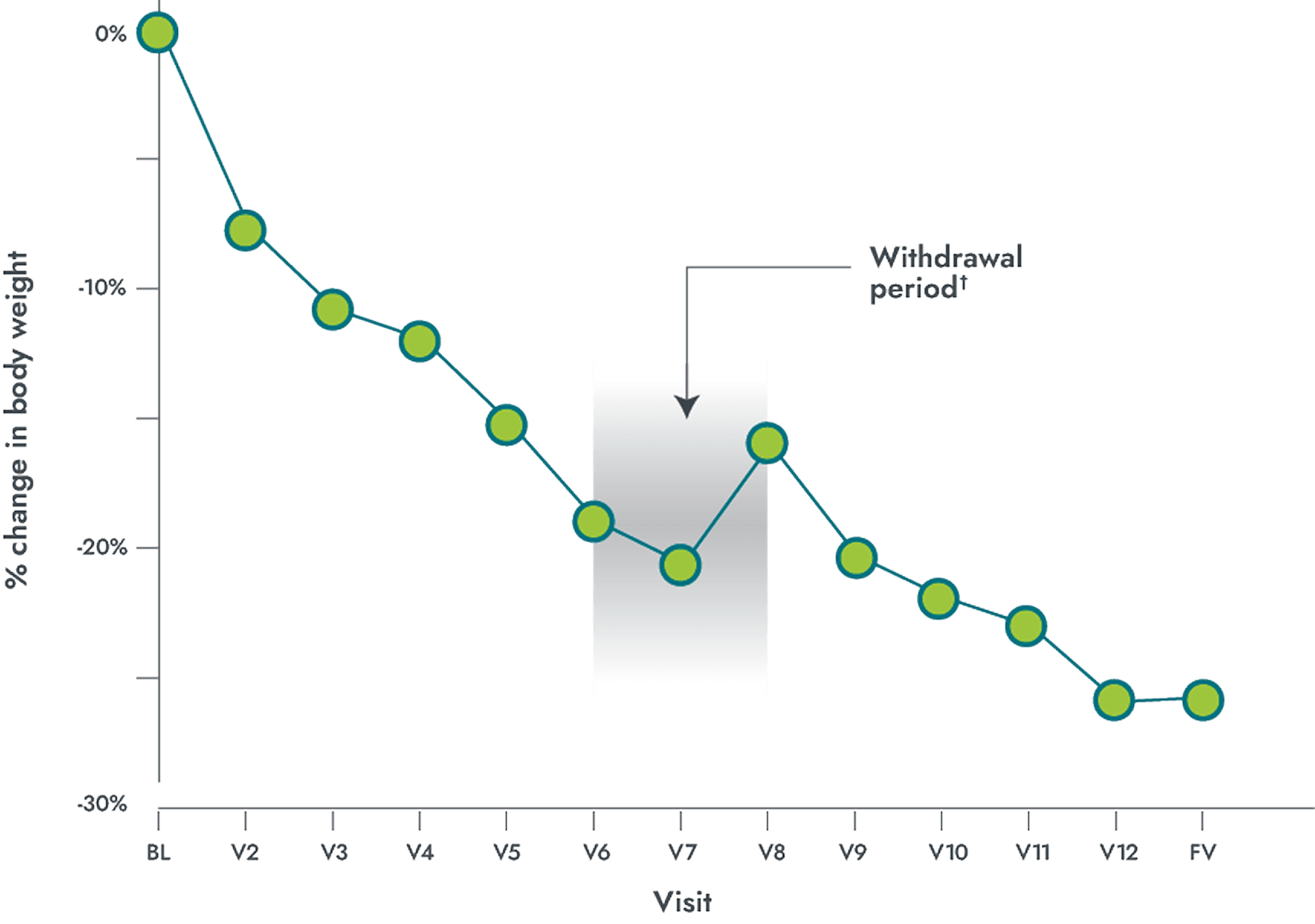
- 80% of patients with obesity due to POMC or PCSK1 deficiency achieved a ≥10% weight loss from baseline after 1 year (primary endpoint) (95% CI: 44.4%, 97.5%); P<0.0001; N=10
- -23.1% mean percentage change in weight from baseline after 1 year (95% CI: -31.9%, -14.4%); P=0.0003; N=10
*Participants who achieved weight loss threshold (≥5 kg or 5% if baseline body weight was <100 kg) during the 10-week open-label period.
†The withdrawal period lasted 8 weeks, which included 4 weeks of IMCIVREE followed by 4 weeks of placebo.
IMCIVREE delivered significant, clinically meaningful weight loss over 1 year in patients 6 years and older with LEPR deficiency
Mean % change in body weight over 1 year
LEPR (n=7)*
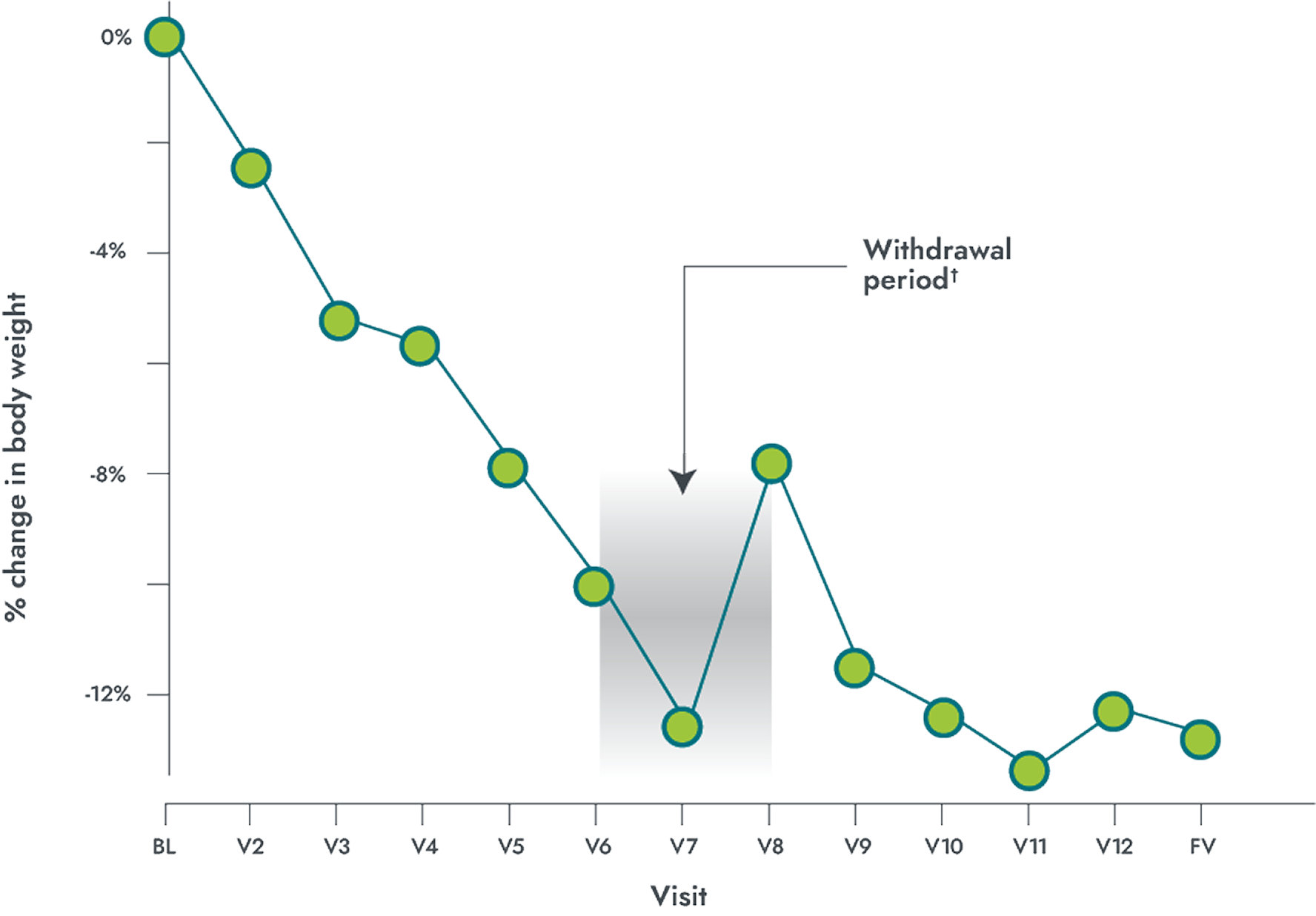
- 45.5% of patients with obesity due to LEPR deficiency achieved a ≥10% weight loss from baseline after 1 year (primary endpoint) (95% CI: 16.8%, 76.6%); P=0.0002; N=11
- -9.7% mean percentage change in weight from baseline after 1 year (95% CI: -16%, -3.3%); P=0.0074; N=11
*Participants who achieved weight loss threshold (≥5 kg or 5% if baseline body weight was <100 kg) during the 10-week open-label period.
†The withdrawal period lasted 8 weeks, which included 4 weeks of IMCIVREE followed by 4 weeks of placebo.
In both studies in patients 6 years and older, weight increased during the withdrawal period, then decreased once treatment was reinitiated1
CI=confidence interval; FV=final visit; LEPR=leptin receptor; PCSK1=proprotein convertase subtilisin/kexin type 1; POMC=proopiomelanocortin; V=visit.
Reference: 1. IMCIVREE [prescribing information]. Boston, MA. Rhythm Pharmaceuticals, Inc. 2. Argente J, Verge CF, Okorie U, et al. Setmelanotide in patients aged 2–5 years with rare MC4R pathway-associated obesity (VENTURE): a 1 year, open-label, multicenter, phase 3 trial. Lancet Diabetes Endocrinol. 2024;24:1-18. doi: 10.1016/s2213-8587(24)00273-0. 3. Data on file. Rhythm Pharmaceuticals, Inc. Boston, MA.